Occurrence
Free, 20% in air, and 0.04% dissolved in water
Preparation
Lab method
1.
By heating a metal carbonate
CaCO3 Δ→ CaO +
CO2↑
ZnCO3 Δ→ ZnO +
CO2↑
CuCO3 Δ→ CuO +
CO2↑ (bluish-green to
black)
Sodium and potassium carbonates do not decompose on heating. Sodium carbonate may give off water vapor.
Na2CO3
· 10H2O → Na2CO3
+ 10H2O
2.
By adding hydrochloric acid to calcium carbonate
CaCO3 + 2HCl → CaCl2 + H2O + CO2 ↑
(Other acids, like
sulphuric acid are not preferred because calcium carbonate gets coated by
another calcium salt, rendering it passive)
The gas is collected under air.
NaHCO3 +
HCl → NaCl + H2O + CO2 ↑
2NaHCO3 Δ→ Na2CO3
+ H2O + CO2↑
Ca(HCO3)2
Δ→ Ca2CO3
+ H2O + CO2↑
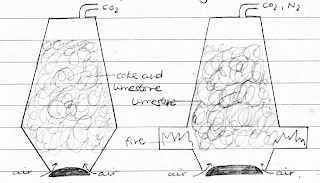
Industrial method
Steam is passed over heated coke. The reaction produces hydrogen and
carbon monoxide. This water gas, if passed with excess steam over a catalyst,
iron (III) oxide, forms carbon dioxide and hydrogen.
C + H2O →
CO + H2
CO + H2 + H2O → CO2 + 2H2
Properties
Physical
1.
It is a colorless, odorless, tasteless acidic
gas.
2.
It is slightly soluble in water. (It can be
dried using P2O5)
Chemical
1.
It does not support combustion, and it does not
burn. However, magnesium burns in CO2 to form its oxide and carbon.
2Mg + CO2 →
2MgO + C
Sodium and
potassium also burn, but later form carbonates.
4Na + CO2
→ 2Na2O
+ C
4K + CO2 → 2K2O
+ C
Na2O + CO2
→ Na2CO3
K2O + CO2
→ K2CO3
2.
It neutralizes alkalies.
CO2 +
2KOH → K2CO3
+ H2O
CO2 +
2NaOH → Na2CO3
+ H2O
CO2 +
Ca(OH)2 →
CaCO3 + H2O
Uses
1.
In fire extinguishers – formed by the action of
sulphuric acid on sodium hydrogen carbonate.
2.
Used as a refrigerant – dry ice.
3.
Is a
leavening agent – baking soda, sodium bicarbonate and an acid in dry form
react.
4.
In soft drinks, dissolved and under pressure.